No products in the cart.
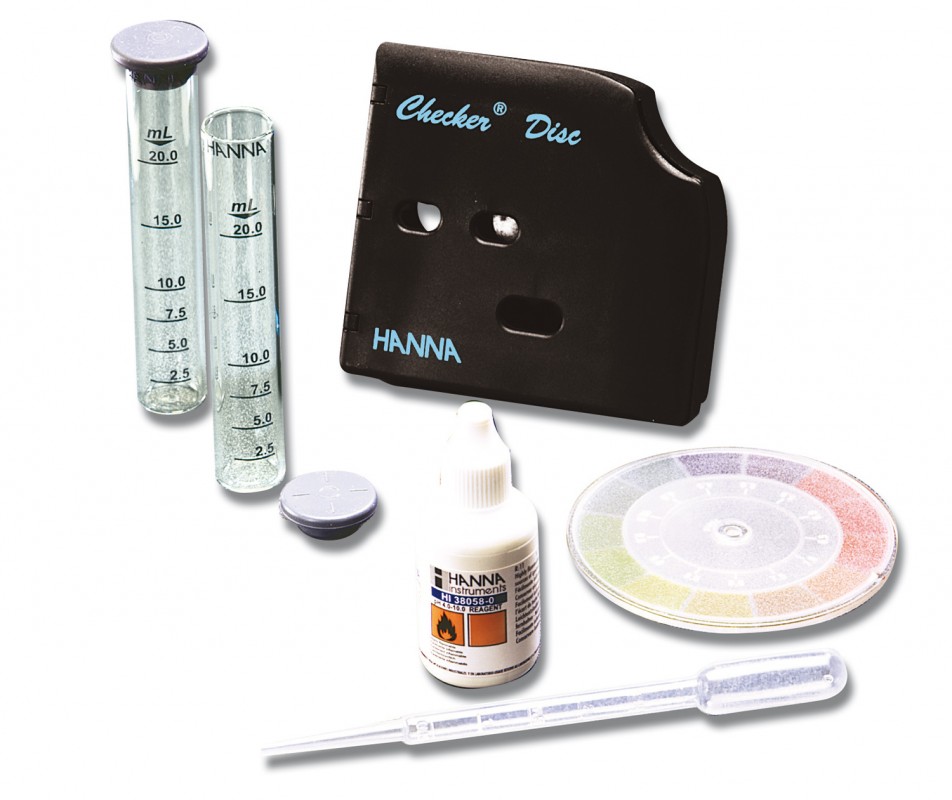
HI38058 CTK pH
฿5,083.57
Chemical test kit for the determination of pH in the range 4-10pH.
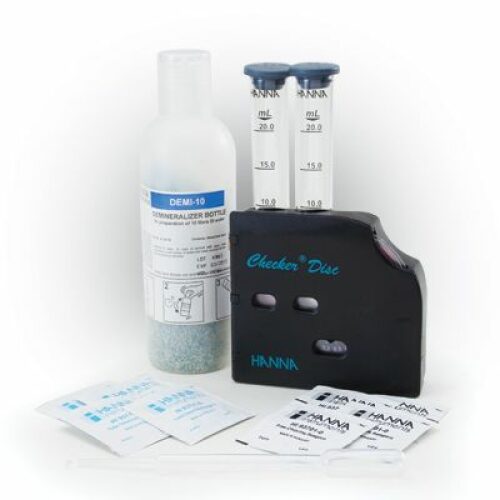
Package size: 300 tests
1 in stock
SKU: HI38058
Categories: All Product, Checker Disc, Colorimetric, Titration, Chemical test kits
pH represents acidity or alkalinity of an aqueous solution and is proportional to the hydrogen-ion concentration of the solution. Under neutral conditions water is dissociated into the (OH)— and H+ ions in equal ratio and hence it has a pH of 7. When bases or acids are added to a water solution they ionize, increasing the concentration of (OH)— or H+, respectively. Thus solutions with a pH of 1-3 contain strong acids, whereas those with a pH of 4-6 contain weak acids.
Weak bases result in solutions of pH 8-10 and strong bases in pH of 11-13.
Order Information:
HI 38058 test kit comes with 30 mL pH 4.0-10.0 reagent, checker disc, glass vials with caps (2) and 3 mL plastic pipette.
Product Manuals
![]() Manual: Download
Manual: Download
Specification :
| Method | Checker Disc | |
| Range | 4.0-10.0 pH | |
| Smallest Increment | 0.5 pH | |
| Chemical Method | pH indicator | |
| Number of Tests | 100 | |
| Weight | 215 g |
Related products
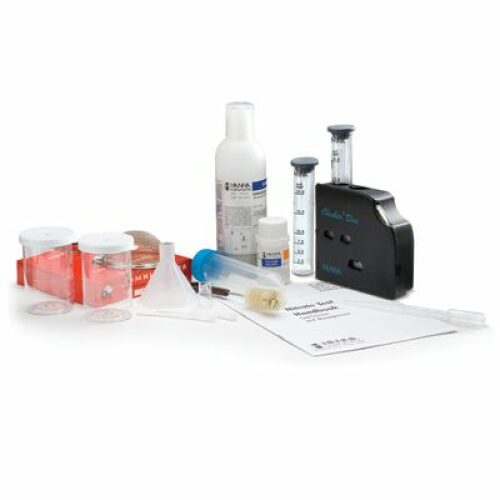
฿7,276.00
Nitrate is reduced to nitrite in the presence of cadmium. The nitrite thus produced reacts with the reagent to yield an orange compound. The amount of color developed is proportional to the concentration of nitrate present in the aqueous sample.
All Product
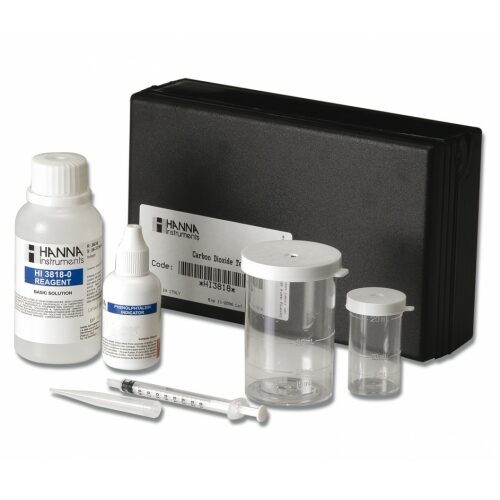
฿1,605.00
Chemical test kit for the determination of carbon dioxide in the range 0 to 10 mg / l / 0 to 50 mg / l / 0 to 100 mg / l (ppm).
All Product
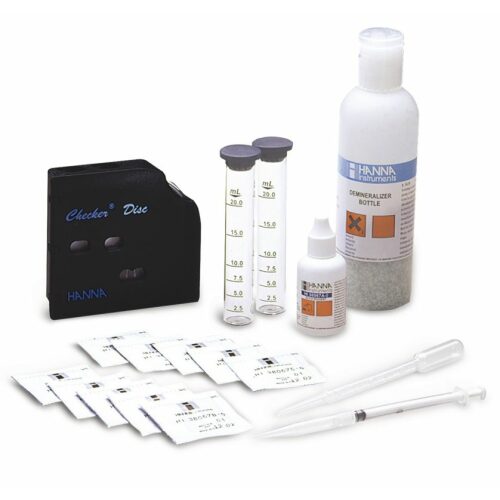
฿7,276.00
Test kit for the chemical determination of silicate in the range 0-40mg / l / 0 800 mg / l (ppm). 100 Tests
All Product
฿4,815.00
Chemical test kit for the determination of free and total chlorine in 0.0-0.70/0.0-3.5 mg / l (ppm).
All Product
฿7,757.50
Chemical test kit for the determination of free and total chlorine in the range 0.0-0.70/0.0-3.5/0.0-10.0 mg / l (ppm). 200 tests
All Product
฿2,461.00
A test kit for the determination of ammonium chemical seawater in the range 0 to 2.5 mg / l (ppm).
All Product
฿2,728.50
Chemischer Testkit zur Bestimmung von Alkalität im Bereich 0 bis 100 mg/l / 0 bis 300 mg/l (ppm). Packungsgröse: 110 Tests