No products in the cart.
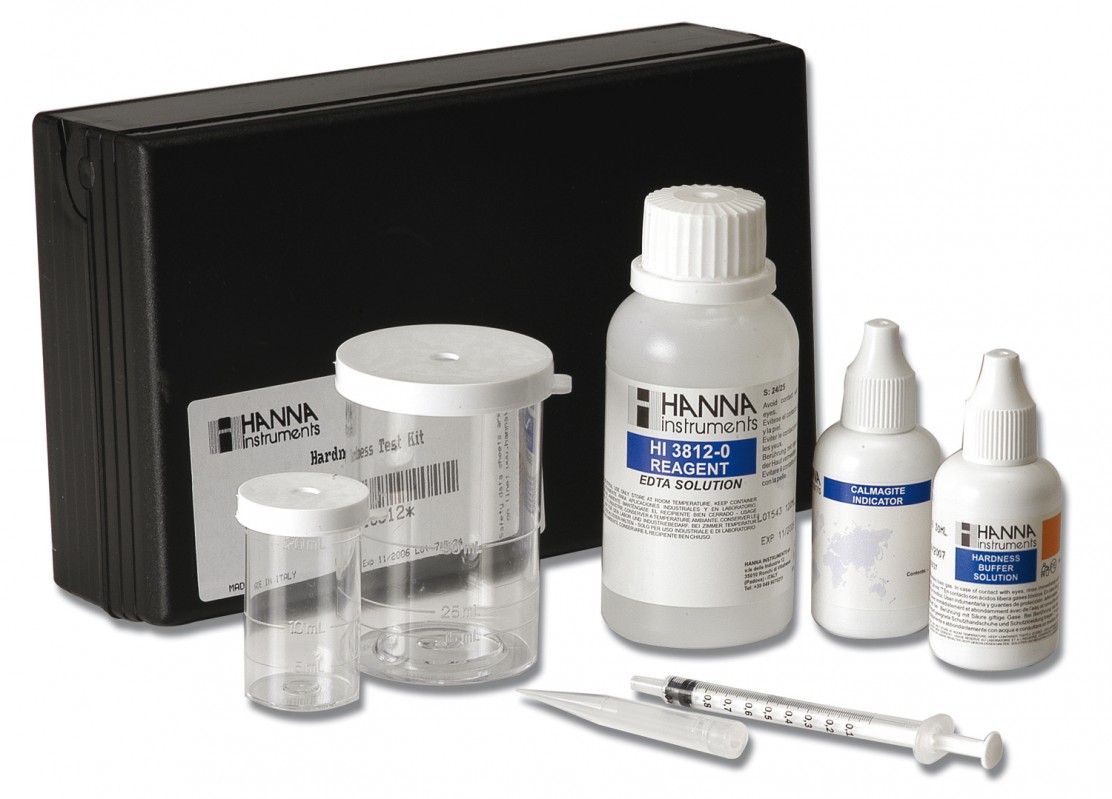
HI3812 CTK Hardness Total
฿1,712.00
Chemical test kit for the determination of total hardness in the range 0.0 to 30.0 mg / l / 0 to 300 mg / l (ppm).
100 Tests
805 in stock
SKU: HI3812
Categories: All Product, Chemical test kits, Titration
Water hardness has traditionally been defined as the capacity of water to precipitate soap. The ionic species in the water causing the precipitation was later found to be primarily calcium and magnesium. In the present, therefore, water hardness is actually a quantitative measure of these ions in the water sample. It is also now known that certain other ion species, such as iron, zinc and manganese, contribute to the overall water hardness. The measure and subsequent control of water hardness is essential to prevent scaling and clogging in water pipes.
The HI 3812 test kit tests the hardness level as mg/L (ppm) calcium carbonate, via an EDTA (ethylene-diamine-tetraacetic acid) titration.
Order Information:
HI 3812 test kit comes with 30 mL hardness buffer, 10 mL calmagite indicator, 120 mL EDTA solution, 20 mL plastic beaker with cap, 50 mL plastic beaker with cap and 1 mL syringe with tip
Product Manuals
![]() Manual: Download
Manual: Download
Specification :
| Method | Titration | |
| Range | 0.0-30.0 mg/L 0-300 mg/L |
|
| Smallest Increment | 0.3 mg/L 3 mg/L |
|
| Chemical Method | EDTA | |
| Number of Tests | 100 | |
| Weight | 460 g |
Related products
All Product
฿6,955.00
Test kit for the chemical determination of silicate in the range 0-1 mg / l (ppm). 100 Tests
All Product
฿5,885.00
A test kit for the determination of chemical copper in the range 0 to 2.5 mg / l (ppm). 100 Tests
All Product
฿5,136.00
A test kit for the determination of chemical sulphite in the range 0 to 20 mg / l / 0 to 200 mg / l (ppm). 110 tests
All Product
฿2,621.50
Chemical test kit for the determination of chloride in the range 0 to 100 mg / l / 0 to 1000 mg / l (ppm).
All Product
฿642.00
Chemischer Testkit zur Bestimmung der Härte im Bereich 0 bis 150 mg/l (ppm). Packungsgröse: 50 Tests
All Product
฿2,728.50
Chemischer Testkit zur Bestimmung von Alkalität im Bereich 0 bis 100 mg/l / 0 bis 300 mg/l (ppm). Packungsgröse: 110 Tests
All Product
฿4,815.00
Test kit for the chemical determination of sulfate in the range 20 to 100 mg / l (ppm). 100 Tests